
| Main centres: | 1-3 business days |
| Regional areas: | 3-4 business days |
| Remote areas: | 3-5 business days |
Potassium (K) fertilizer is commonly added to improve the yield and quality of plants growing in soils that are lacking an adequate supply of this essential nutrient. Most fertilizer K comes from ancient salt deposits located throughout the world. The word "potash" is a general term that most frequently refers to potassium chloride (KCl), but it also applies to all other K-containing fertilizers, such as potassium sulfate (KSO, commonly referred to as sulfate of potash, or SOP).
Production
Potassium is a relatively abundant element in the Earth's crust, and production of potash fertilizer occurs in every inhabited continent. However, KSO is rarely found in a pure form in nature. Instead it is naturally mixed with salts containing magnesium, sodium and chloride (Mg, Na and Cl, respectively). These minerals require additional processing to separate their components. Historically, KSO was made by reacting KCl with sulfuric acid. However, researchers later discovered that they could manipulate a number of earth minerals to produce KSO, now the most common method of production. For example, natural K-containing minerals (such as kainite and schoenite) are mined and carefully rinsed with water and salt solutions to remove byproducts and produce KSO. The mining industry uses a similar process to harvest KSO from the Great Salt Lake in Utah and from underground mineral deposits.

In New Mexico, KSO is separated from langbeinite minerals by reacting it with a solution of KCl, which removes the byproducts (such as Mg) and leaves KSO. Similar processing techniques are used in many parts of the world, depending on the raw materials accessible.
Agricultural use
Potassium is needed to complete many essential functions in plants, such as activating enzyme reactions, synthesizing proteins, forming starch and sugars, and regulating water flow in cells and leaves. Often, concentrations of K in soil are too low to support healthy plant growth.
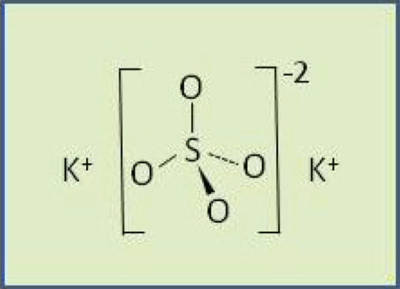
Potassium sulfate is an excellent source of K nutrition for plants. The K portion of the KSO is no different from other common potash fertilizers. However, it also supplies a valuable source of S, which protein synthesis and enzyme function require. Like K, S can also be too deficient for adequate plant growth. Further, Cl- additions should be avoided in certain soils and crops. In such cases, KSO makes a very suitable K source.
Potassium sulfate is only one-third as soluble as KCl, so it's not as commonly dissolved for addition through irrigation water unless there's a need for additional S.
Several particle sizes are commonly available. Manufacturers produce fine particles (smaller than 0.015 mm) to make solutions for irrigation or foliar sprays, since they dissolve more rapidly. And growers find foliar spraying of KSO a convenient way to apply additional K and S to plants, supplementing the nutrients taken up from the soil. However, leaf damage can occur if the concentration is too high.
Management practices
Growers frequently use KSO for crops where additional Cl from more common KCl fertilizer is undesirable. The partial salt index of KSO is lower than in some other common K fertilizers, so less total salinity is added per unit of K. The salt measurement (EC) from a KSO solution is less than a third of a similar concentration of a KCl solution (10 millimoles per liter). Where high rates of KSO are needed, agronomists generally recommend applying the product in multiple doses. This helps avoid surplus K accumulation by the plant and also minimizes any potential salt damage.
